Background:
Acute myeloid leukaemia (AML) is a blood cancer characterised by the expansion of a malignant myeloid progenitor. The estimated age-standardised incidence rate in many western countries has remained static over the last 2 decades at 3-4 per 100,000, whilst long-term survival has improved, especially for the younger individuals. However, disparities remain for the older individuals, people of ethnic minority background, and those who are more socio-economically deprived. Previous evaluation of population data from New Zealand has shown a similar pattern, but a more recent analysis has not been done. Here we present the incidence and long-term survival of patients with AML in New Zealand (NZ), using the New Zealand Cancer Registry (NZCR).
Method:
The NZCR was established in 1948 and it became mandatory by law to report all new cases of malignancy by 1994. We extracted all AML cases from the registry between 1 January 1997 and 31 December 2016. Cases with an ICD-10-CM code for acute myeloid leukaemia and its subtypes including acute promyelocytic leukaemia (e.g. C92.0) were included. Individuals residing overseas or without an address were excluded, and individuals with a diagnosis of acute promyelocytic leukaemia (APML) were analyzed separately. The socio-economic status of the individual was estimated based on their domicile area using the New Zealand 2013 Index of Deprivation (NZDep2013) which is a geographically based composite measure of deprivation. Overall survival was calculated from the date of diagnosis to the date of death or last follow-up (31 December 2016). Multivariable Cox-proportional hazard models were used to evaluate potential associations with survival time in NZ AML cases.
Results:
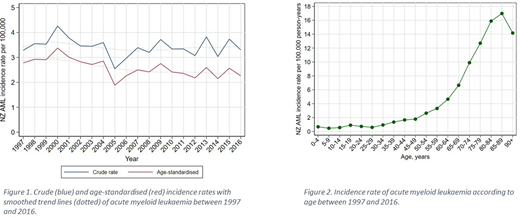
During this 20-year period, 154 cases of APML and 2876 cases of AML (excluding APML) were reported to the registry on individuals residing in New Zealand. Of the AML cases, 53% were male and the median age at the time of diagnosis was 67 (IQR 52-77), with a small positive correlation between year of diagnosis and age at diagnosis (Spearman's rho=0.05, p=0.009). The majority of cases (77%) were of European descent, 12% were New Zealand Maori, and 6% were Pacific Islanders. Individuals of European descent were significantly older at diagnosis compared to other ethnicities (median of 70 vs 51 for Maori, 56 for Pacific Islanders, and 58 for all other ethnicities, p<0.001). AML appeared to disproportionally affect those more socio-economically deprived, with 23% of cases reported in the most deprived 20% of the population, compared with only 16% of the cases in the least deprived 20%. The annual crude incidence remained stable during this period at an average of 3.42 per 100,000 (ranging from 2.57 to 4.29, figure 1), and was significantly higher in the older adults (figure 2). Age-standardised rates were lower (figure 1), with an average of 2.6 (range 1.9 to 3.4) cases per 100,000, and a small but significant average annual decrease over the study period. The estimated 1, 2, and 5-year survival for the entire cohort was 38%, 27%, and 22%, respectively. Age at diagnosis was a significant predictor of inferior survival, with a hazard ratio (HR) for all-cause mortality of 2.06, 3.95, 6.39 and 10.84 for the 50-59, 60-69, 70-79 and >80 age groups, respectively, compared to those aged <50. Shorter overall survival was also noted in individuals in the more socio-economically deprived 50% of the population (HR 1.13, 95% CI 1.03-1.23).
Conclusion
The incidence of AML in New Zealand has remained static in the last 2 decades, consistent with data from other western countries. Lower age-standardised rates and the small decrease in these observed over the study period are likely to reflect the increasingly and comparatively older population in NZ. Maori and Pacific Islanders appeared to present at a younger age than individuals of European descent. Age at diagnosis and socio-economic deprivation were shown to be an adverse prognostic factor for overall survival. Further in-depth analysis is required to determine the cause of these observations at a population level.
Chan:AbbVie:Membership on an entity's Board of Directors or advisory committees;Janssen:Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau;Celgene:Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company);Amgen:Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company);Roche:Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal